Host specificity of Avipox viruses: An experimental study with field isolates of fowlpox, pigeonpox and duckpox viruses Pathak N.*,3, Baruah G.K.3, Pathak D.C.3, Upadhyaya T.N.3, Barman N.N.1,3, Kalita N.2,3, Deka P.1,3, Gogoi S.M.1,3 3Department of Veterinary Pathology, College of Veterinary Science, Assam Agricultural University, Khanapara, Guwahati-781022, Assam, India 1department of Veterinary Microbiology, College of Veterinary Science, Assam Agricultural University, Khanapara, Guwahati-781022, Assam, India 2department of Poultry Science, College of Veterinary Science, Assam Agricultural University, Khanapara, Guwahati-781022, Assam, India *Corresponding author: e-mail: drnayanjyotipathak@gmail.com
Abstract A study was conducted to determine the host specificity of field isolates of fowl pox virus (FPV), pigeon pox virus (PPV) and duck pox virus (DPV) by giving cross infection. During inoculation of fowl pox virus (FPV) in embryonated chicken eggs, and duck pox virus (DPV) in embryonated duck eggs showed PCR positive during the first passage itself. Inoculation of pigeonpox and duckpox viruses in embryonated chicken eggs required 2–3 initial passages to get the positive results. In the experimental study, all the chicks inoculated with FPV developed pox lesions within 7–9 days post infection. Similarly, all the pigeons inoculated with PPV developed pox lesions within 5–9 days. Likewise, all the ducklings inoculated with DPV developed pox lesions within 5–6 days. In cross infection study when FPV, PPV and DPV were inoculated in heterologous hosts no lesions were developed. Histopathological examination of the scab samples of infected birds revealed intracytoplasmic eosinophilic inclusion bodies. Among the visceral organs the lungs showed congestion and haemorrhages with proliferation of lymphoid cells around the bronchioles, the heart showed capillary congestion between the myofibrils, hydropic degeneration and fatty changes in the liver and renal tubular epithelium. The scab samples of infected birds showed positive results for APV by PCR; but the biopsy samples collected from the inoculated site of the heterologous hosts showed PCR negative. It was concluded that field isolates of fowl pox virus (FPV), pigeon pox virus (PPV) and duck pox virus (DPV) exhibited host specificity and did not produce cross infection in heterologous hosts. Top Keywords Fowlpox virus, Pigeonpox virus, Duckpox virus, Host specificity. Top |
INTRODUCTION Poultry, pet and different wild birds are commonly affected by Avipoxvirus infection resulting mortality in young ones and retarded growth in adult birds. Avipoxvirus infection has been reported to affect over 232 species in 23 orders of birds1. Avipoxviruses are usually named on the basis of the bird species from which the virus was first isolated and characterized2. Among different Avipoxviruses of domestic birds, fowlpox virus (FPV) and pigeonpox virus (PPV) infection is found to be very common, whereas, records related to the incidence of duckpox (DP) is very scanty. There is much confusion regarding the incidence of pox in domestic ducks. It is because according to Ward and Gallaghar3, the avian pox does not occur naturally in ducks, whereas Te Hennepe4 reported the occurrence of fowlpox (FP) in ducks during the year 1924. |
As the clinical and pathological manifestations of pox virus infection in fowl, pigeon and duck are similar to each other, there may be chance of cross infection, because at many places fowls, pigeons and ducks are reared in the same house or in same location by different households. Therefore, the present study was undertaken with an attempt to determine the host specificity of FPV, PPV and DPV isolates prevalent in Assam. |
Top MATERIALS AND METHODS Isolation of wild strains of Avipoxvirus Among various field isolates of FPV, PPV and DPV, one representative pox positive isolate from each species was used for the present study. The virus isolates of fowlpox (F3) and pigeonpox (P2) were adapted on chorioallantoic membrane (CAM) of embryonated chicken eggs, whereas duckpox (D1) isolate was adapted on CAM of embryonated duck eggs. Representative pieces of CAMs were stored at −20°C for the future use and in 10% formalin solution. Calculation of EID50 The EID50 was calculated in case of fowl, pigeon and duck eggs by following Reed and Muench method5. The eggs were incubated at 37°C up to a period of 7 days and observed daily for embryopathy. The embryos after each death as well as those survived up to 7 days were harvested. All the CAM were examined and the lesions were recorded properly. These lesions were confirmed clinically as well as by PCR. From the positive and negative cases the EID50 were calculated from eggs of all the 3 species. A total of 15 numbers of 4 week-old healthy Kamrupa chicks (a newly developed crossbred chicken), pigeons and ducklings were used for the experiment. The birds were kept in separate houses by following proper managemental condition and the experimental infection was given after one week of adaptation. During this period no vaccination and other medication were used. The experimental birds of each group were inoculated with virus suspension, where the virus concentration was 104.5 EID50, 105.24 EID50 and 104.83 EID50 of FPV, PPV and DPV, respectively as shown in Table 1. The suspension was put on the scratched surface of the skin of thigh region and/or defeathered areas under the wings. Clinical Observations The chicks and pigeons were observed for 5 weeks whereas ducklings were observed only for 3 weeks post infection for development of any external lesions and later sacrificed. Scab samples collected from clinically affected and samples from inoculation site of unaffected fowls, pigeons and ducklings were processed for detection of viral nucleic acid by PCR. The PCR was performed using a primer pair described by Lee and Lee6 based on 4b sequence of fowlpox virus strain HP444 as: Forward primer: 5‣- CAGCAGGTGCTAAACAACAA-3‣ Reverse primer: 5‣ - CGGTAGCTTAACGCCGAATA-3‣ Gross and histopathological study The cutaneous lesions of the affected birds and portion of the visceral organs of all the affected and unaffected birds were collected in 10% formalin solution and processed as per standard procedure7 for histopathological study. The paraffin embedded tissues were cut into 4–5 μm thick sections and stained with H &E stain. Top RESULTS Isolation of the Avipoxvirus and host specificity in embryonated eggs Out of the various samples, 10 nos. of FPV, 6 nos. of PPV and 3 nos. of DPV were isolated. When FPV was inoculated in embryonated chicken eggs then 100% recovery was seen at the first passage itself, but in case of PPV inoculated in embryonated chicken eggs 100% recovery was seen at the second passage. Similarly, when DPV was inoculated in embryonated chicken eggs only 66.66% recovery was observed in fifth passage, but in embryonated duck eggs 100% recovery was seen at the first passage itself (Table 2). Grossly, characteristic pock lesions were observed in the positive cases on CAMs (Fig. 1). Initially at the first or second passage, the CAMs appeared opaque, leathery, edematous and thick. In third-forth passage opaque raised necrosed areas were observed in the CAMs. Histopathological examination of the infected CAMs revealed hyperplasia and hyperthrophy of the epithelial cells, making the CAM thicker than normal. In the mesoderm the blood vessels were seen severely congested. There was hyperplasia of the epithelial cells of the allantoic membrane forming many layers at some places, which was a common observation. The hyperplastic epithelial cells showed hyperchromatic nuclei with faintly stained cytoplasm (Fig. 2). Host specificity in fowls, Pigeons and ducks In chicks (Experiment-I): From the study it was observed that all the chicks inoculated with FPV by scratching over the skin developed characteristic pox lesions (pustules) within 7–9 days post infection and showed positive results through PCR. On the other hand, when the chicks inoculated with PPV and DPV none of the chicks developed any gross lesions and all the chicks showed negative results through PCR (Fig. 7). In pigeons (Experiment-II): Similarly, all the pigeons inoculated with PPV showed the characteristics pox lesions within 5–9 days and none of the pigeons inoculated with FPV and DPV showed any pox lesions and were negative through PCR. In ducks (Experiment-III): In this experiment all the ducklings inoculated with DPV showed grossly visible pox lesions within 5–6 days and were positive through PCR and none of the ducklings showed pox lesions when inoculated with FPV and PPV and were negative through PCR. Gross pathology Characteristic black to brown coloured nodular growths on scratched areas were seen in all the infected chicks, pigeons and ducklings which later developed into scabs and detached from the body after 22 days onwards in case of chicks, 27 days onwards in case of pigeon and 10 days onwards in ducklings. No significant gross lesions were observed in any visceral organ. Microscopic Pathology Histopathological examination of the collected scab samples of chicks, pigeons and ducklings revealed epithelial hyperplasia, hypertrophy, ballooning degeneration, encapsulation by fibrous tissue capsule and presence of intracytoplasmic eosinophilic inclusion bodies in the epithelial cells. The inclusions were of various sizes and some of them occupied major portion Top DISCUSSION Variation in propagation of virus in heterologous hosts indicated time requirement for adaptation in cell receptors. There may be species specificity in case of pigeonpox and duckpox for their adaptation in chicken embryo which was also supported by Holt and Krogsrud8 who observed that some strains of APV did not grow in chicken embryos. From the present study it is revealed that different APVs take time to adapt in the heterologous host. |
The gross lesions observed in pox infected CAM were edema, necrosis, hyperplasia and hypertrophy of the epithelial cells of the CAM making it thick, leathery and opaque. These changes were similar to the observations described by earlier workers in FPV infection9. The observations of black to brown nodular growths on the scratched areas in all the infected fowls, pigeons and ducks were in agreement with previous natural as well as experimental studies10,11,12,13-14. Though there was no mention about the morphological alterations in visceral organs in different studies12,15,16, the present histopathological findings in some visceral organs showed some alterations which might be due to viral growth in certain organs causing secondary viraemia as described by earlier17. Microscopic findings of the scab samples showed epithelial hyperplasia, hypertrophy, ballooning degeneration, encapsulation by fibrous tissue capsule and presence of intracytoplasmic eosinophilic inclusion bodies considered to be pathognomonic lesion of FP, corroborating the observations of earlier workers18,19. Previous studies13 also described histopathological lesions in visceral organs of pox infected pigeons as oedema with fibrin deposition in alveolar spaces and huge infiltration of heterophils in interstitial spaces of lungs. In the present study lymphoproliferation in the peribronchial areas was also noted. |
It was concluded from the study that field isolates of fowl pox virus (FPV), pigeon pox virus (PPV) and duck pox virus (DPV) exhibited host specificity and did not produce cross infection in heterologous hosts. |
Top ACKNOWLEDGEMENTS The author likes to thank Department of Microbiology, personnel of the Central Instrument Facility and Advance Animal Disease Diagnosis and |
Management Consortium” (ADMaC), College of Veterinary Science, Assam Agricultural University, |
Khanapara, Guwahati-781022 for helping him to conduct this research as a part of his Ph D degree. |
Top Figures | Fig.1.: Opaque, leathery, edematous and thick CAM with pock lesions (arrow);
| 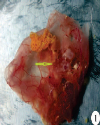 | |
| | Fig.2.: CAM showing hyperplasia of the allantoicmembrane epithelium and congested blood vessels in the mesoderm with faintly stained cytoplasm. H&E ×400;
|  | |
| | Fig.3.: Fibrous tissue encapsulation of pox nodule, hyperplasia and intracytoplasmic degeneration and inclusion bodies inclusion bodies. H&E ×100;
|  | |
| | Fig.4.: Vacuolation with ballooning in the epidermal cells. H&E ×400;
|  | |
| | Fig.5.: Lung showing lymphoid nodule H&E ×400;
|  | |
| | Fig.6.: Fatty changes and hydropic Degeneration in the epithelial cells of the distant convoluted tubules of kidney (arrow). H&E ×400.
|  | |
| | Fig.7.: Agarose gel electrophoresis photograph showing amplicons. Lane 1: Positive control (fowl pox), Lane 2: Pigeon pox virus isolate, Lane 3: Duck pox virus isolate, Lane 4: Negative sample, Lane 5: Negative control, Lane 6: Ladder (100 bp) of the epithelial cell cytoplasm (Fig. 3,4). In the lungs congestion and haemorrhage with proliferation of lymphoid cells in follicular pattern were seen mainly around the bronchi and bronchioles along with mild hyperplasia of the bronchial epithelium (Fig. 5). The liver and kidneys showed hydropic degeneration and fatty changes (Fig. 6).
|  | |
|
Tables | Table 1.: Experimental design to determine host specificity of FPV, PPV and DPV.
| | Experiment No. (Host) | Group of birds | No. of birds | Virus isolates | Virus titre (log 10 ID50/0.1 ml) | | I(Chicks) | I | 5 | FPV | 4.50 | | II | 5 | PPV | 5.24 | | III | 5 | DPV | 4.83 | | II(Pigeons) | I | 5 | FPV | 4.50 | | II | 5 | PPV | 5.24 | | III | 5 | DPV | 4.83 | | III(Ducklings) | I | 5 | FPV | 4.50 | | II | 5 | PPV | 5.24 | | III | 5 | DPV | 4.83 |
| | | Table 2.: Isolation of Avipox viruses in homologous as well as heterologous hosts at various passage levels.
| | Isolates | No. of sample | Type of Embryo used | No. of Positive isolates (% positive) at different passage levels | Recovery percentage | | 1st | 2nd | 3rd | 4th | 5th | | FPV | 10 | Fowl | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 100 | | PPV | 6 | Fowl | 1 (16.66) | 6 (100) | 6(100) | 6(100) | 6(100) | 100 | | DPV | 3 | Fowl | 0 | 0 | 1 (33.33) | 2 (66.66) | 2 (66.66) | 66.66 | | DPV | 3 | Duck | 3(100) | 3 (100) | 3(100) | 3(100) | 3(100) | 100 |
| |
|